Combustible fuels have been around ever since humans realized that they could burn wood. Over time, we discovered new energy sources—fossil fuels, which still dominate the world’s energy portfolio. But what if we could find another fuel source, one that was abundant, easy to procure, and people were happy to give it away? Something like municipal or agricultural waste? One fuel, called hydrochar, which is a proposed replacement for coal, can be made in a laboratory from waste using water and some low-cost catalysts. It might just be the answer to a burning energy question.
Transcript
INTRO: Electricity or batteries are really good for some applications. But they're not going… they're not going to solve all the problems. And so the question is really how do we make energy, chemical energy carriers, or fuels as, efficiently as possible utilizing those waste streams? And then how do we use all the good work that people have done upstream and actually burn the fuel as efficiently as possible?
HOST: Welcome to Growing Impact, a podcast by the Institutes of Energy and the Environment at Penn State. Growing Impact explores cutting-edge projects of researchers and scientists who are solving some of the world’s most challenging energy and environmental issues. Each project has been funded through an innovative seed grant program that is facilitated through IEE. I’m your host, Kevin Sliman.
HOST: Combustible fuels have been around ever since humans discovered they could burn wood. Over time, we discovered new energy sources—fossil fuels, which still dominate the world’s energy portfolio. Today, scientists are working to develop new cleaner and more efficient fuels. On this episode of Growing Impact, I speak with Jacqueline O’Connor, an associate professor of mechanical engineering at Penn State. She and her team are working on how to turn agricultural and municipal waste into bioproducts, including fuels that can have a net-negative carbon footprint.
HOST: Welcome, Jacqueline, to Growing Impact.
Jacqueline O’Connor (JO): Thanks Kevin. I'm really excited to be here.
HOST: Can you introduce yourself and just give us a taste of your research background and expertise?
JO: My name is Jacqueline O'Connor. I'm an associate professor of mechanical engineering. I started here at Penn State in August of 2013. Before coming here, I did a PhD at Georgia Tech and then was a postdoctoral researcher at Sandia National Labs for a little while and then came here to Penn State. So this is my 10th year at the University. My background is in engine combustion. So I did my PhD in gas turbines in this phenomenon that like 50 people on planet Earth really care about called thermoacoustics. It's the interaction between flames and sound. But I've always really been motivated to study engine combustion because that's the source of the emissions problem.

HOST: Could you talk a little bit about the goal of your project and maybe some of its background?
JO: The current project that I'm working on now is looking at combustion characteristics of hydrochars. These are solid alternative fuels that have been proposed for a replacement for coal in applications where it's useful to have a solid fuel, either because of transport limitations or because you want a fuel that radiates. A lot of industrial processes either rely on the chemistry of the hydrocarbon fuel or just need a fuel that produces a crazy amount of heat, both in terms of high temperature and in terms of radiation. And that's where solid fuels can be really useful. But we'd like to replace coal and these hydrochars, depending on how you make them and how you source them, can actually be a net negative carbon source of fuel. This seed grant is a collaboration between myself doing the experiments; Dr. Jonathan Mathews, who is a professor of energy and mineral engineering here at Penn State. He has a really amazing simulation tool that we're going to be using or we are using; and then we have a collaborator at Cornell, my good friend Jillian Goldfarb, and she's the one who's actually making the fuels.

HOST: What are some challenges that need to be addressed for biofuel products?
JO: So the goal of this project is to better understand the structure of solid fuels that are made through a process called hydrothermal carbonization. So if you think about raw biomasses, and this could be things that are grown as a feedstock, they could be waste biomasses like corn stover, or they could be even like human-generated wastes, like municipal solid waste or restaurant waste or coffee grounds or something like that. All of these have relatively low-energy density because there's so much other stuff in plants. So you can enhance the energy density of biomass fuels by thermally treating them. And there are three different ways that we do this. One is called pyrolization, so basically you make charcoal out of it. This is sometimes also referred to as torrefaction. You can gasify it, so that's—pyrolization typically doesn't have any oxygen present, it just carbonizes everything. And we've all seen a charcoal briquette, that comes from a plant and then it becomes this black solid thing. You can gasify it, which is a really good way to get the hydrogen and smaller molecules out of it. Or you can use a process called hydrothermal carbonization, where you're basically, you're taking the process that the Earth used to make coal, but you're speeding it up in a laboratory over a couple of hours. So you put this stuff in water at high pressures and moderate temperatures, right near where water becomes a supercritical fluid. And water is a really weird fluid to start out with. And near its supercritical point, it gets even weirder, it becomes a really great solvent. And it allows all of these carbonization reactions to occur that wouldn’t occur in a highly polarized water environment at regular temperatures and pressures. So with a few surprisingly cheap catalysts, no rare earth metals or anything, you can get really high-value, high-density, energy density products out of this process in a few hours with a relatively low energy input.
HOST: What affects how these fuels turnout?
JO: Depending on the input stream and how you cook it, basically, you can get really different structure. There are two parts to these fuels. There's the main carbon char, and then there's these little globules that end up on the outside, and they have a really different chemical composition. We think they're going to ignite really differently. And so we're trying to understand them. So that is the context of the larger project.
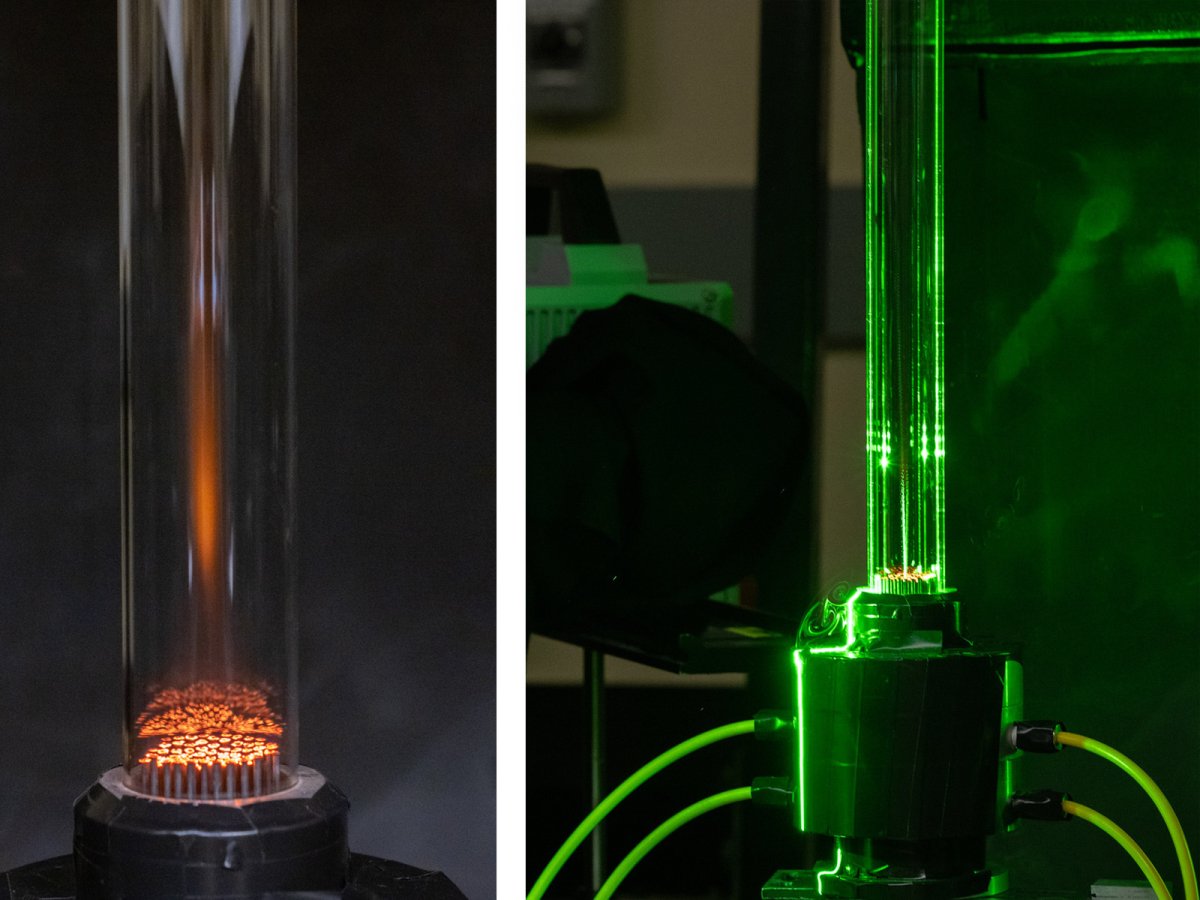
The project specifically for the seed grant, right now, I have this ongoing collaboration with my wonderful colleague at Cornell, Jillian Goldfarb. She makes the fuel, and then I burn it. It's a great friendship. We actually met at a wine and cheese night, and we were just talking, and so we were friends and then we figured out what each other did. And it was like, "Oh, we should work together. This is perfect." But Jonathan Mathews is the other part of this, and he has a really neat technique to model carbonized materials. He's traditionally used it for coal but wants to extend it for these biomass-based chars. And what we're really interested in is what their structure is and basically how gas can get in and out of that structure.
The first step in burning any solid or carbonaceous solid, I should say, is that it kind of off gases. If you've ever sat in front of a fire, like a campfire, you can hear it kind of hiss and pop. Those are gases escaping from the wood itself in a process called devolatilization. So those volatile gases are actually what's burning first, and the ease with which a char can devolatilize is probably the biggest controlling timescale for how fast it's going to burn, how complete it's going to burn, what its emissions are going to be. And so we want to better understand the actual structure so we can better understand our experimental data, and when we measure things like ignition delay time.

HOST: How do Jonathan's models assist in this process?
JO: The cool thing about these models is that he can take images from transmission electron microscopy and actually read the image and understand the structure using some really advanced image analysis tools. And then turn that into a molecular model of the char itself. He can put the gaps in, he can put in all those internal passages where the gas is going to escape. And then we can look at the structure, and he's actually done some work previously to start to calculate how you would predict these devolatilization rates from understanding the structure. The seed grant is really providing us funding to do two things. And for my group, this is all totally new. For Jonathan's group, this is an extension of a tool that they already have. We'll be doing the microscopy. We've never done microscopy before—my grad students learning how to use all these machines at the MCL (Materials Characterization Lab), which is excellent. We'll have really good images, which in and of itself will be helpful, but then there'll be the input to the model so that we can understand transport—particularly mass transport—better in these solids.
HOST: Are you finding that Jillian’s work to either Jonathan's or yours, and then yours then to Jonathan's, are they then feeding back and forth to each other like you learn something and then that goes back to Jillian, and she thinks about a way, a new way to build, to create the fuel or the bioproduct? Are you informing one another back-and-forth, essentially?
JO: Very much so. We're still at the beginning. But that's, that's very much the a) it's a great team and we work really well together. And our students are really great and working well together. So that just sort of organically comes up in the discussion. But yes, that's very much it. Especially I think the insights that we're going to get from the results of the modeling, which we're just starting. That's going to tell us much more about how to tune the process. Because you get such a, I call it like a big number out of combustion, you get an ignition delay time, which is dependent on six zillion things, right? But the models are going to give us much more detailed numbers and quantities that then we'll be able to tune the process.
HOST: This project is not only creating potential new fuels for the future, but it's removing wastes from the waste stream. So it's a two-for-one, if you will. It's two benefits out of one potential, in one project, I guess I'll say.
JO: Yeah, absolutely. And that sort of twofer that you get is one of the, both benefits, but also challenges of alternative fuels. There are a lot of waste streams that one could capture, and there are a lot of different upgrading processes that you could use, right? So another example that I use of animal waste lagoons, they're also really good options for anaerobic digestion, right, to make renewable natural gas. I'm sure they could be used for other things, natural fertilizers and—I don't really know that much about poop, so that took my understanding. But what I do know, there are a lot of different ways that you could use these waste streams. And so one of the challenges of alternative fuels that we're not tackling in this grant, but I certainly think about in motivating the work that I do—is yes, you have all these waste streams, but they have a lot of different ways that they could be used. How do you balance out both the carbon benefit of using the waste streams and the final product, as well as the economic benefit to the farmer or to the company that's making the product or anything like that.
HOST: What are some of the facets that you need to be mindful of when developing new fuels?
JO: One of the other things that you really need to consider is how well these things burn. We have a lot of experience burning coal. And there is a shocking amount of fundamental scientific research that's still happening on making that process more efficient. The energetic content of a renewable fuel, its physical structure, its chemical structure, how it moves through the air, how these diffusional and transport processes happen on a very small scale. All of these can have large-scale effects on the operability and performance of a big furnace system that would—or a boiler system—that would actually burn these fuels.
I think one of the things that we’re really motivated by on the combustion side of the work is you can't just switch these things out, right? You can't take coal out and put hydrochar in. People have done studies of cofiring with coal and biomass, and coal and other thermally processed biomasses. They just burn really differently. If you have a system that's really tuned to burn coal efficiently, then it's probably not going to burn this hydrochar efficiently. And that's bad, right? We just spent all this time trying to make the process of making these things with the lowest carbon intensity possible. And then if you just shove them in a combustion system that wasn't designed for them, you're going to lose a lot of those benefits. The work that Jonathan and I are doing is really focusing on the burning part, so that we have a better idea of how to implement this fuel successfully into an existing system.
HOST: What are some challenges that need to be addressed for biofuel products?
JO: There are a number of challenges in adopting bioproducts, biofuels, in particular. I think they fall into three categories. One is just this source of the feedstock and where it's going to come from and what else it can be used for. I think the second challenge is scaling up the conversion process. If we're going to replace large amounts of hydrocarbon fuels with these thermal processes, the scaling, the efficiency, and the scaling the infrastructure, all those sorts of things, are extremely challenging. And many of the challenges have nothing to do with science, right? They have a lot to do with economics and incentives and taxes and things like that. The third challenge is, on the utilization side. Do the devices that we're using fuels in, are they fuel flexible? That's the word that we use in the biz. And there are certain devices, particularly newer ones, where folks have designed combustion systems knowing like, okay, we need this to be fuel flexible and still really efficient. You can get older devices to be fuel flexible. They just won't be as efficient. And in our world, efficiency equals CO2 output.
The thing that I am trying to do in my own work and in my engagement with IEE is to talk to the people who are at each step of that process, to spend time with agricultural engineers who are thinking about feedstocks so that they know at the end of the chain what we're thinking about on the device and utilization side, to partner really tightly with folks who are doing the synthesis so we can have that feedback between combustion performance and synthesis. So that's one of the benefits I think of working with IEE and having such wonderful colleagues at a university like Penn State, where we have folks thinking about the plants and the farmers and the supply chains. The people in the middle on the synthesis side and then the people on utilization side.
HOST: How will bioproducts ultimately improve our world?
JO: Bioproducts improve the world from a carbon perspective in two big ways. One, they provide a pathway for wastes to be utilized. Then the second great benefit is replacing fossil fuels with lower carbon intensity fuels. And I strongly, strongly believe that chemical-based energy carriers will still be necessary for at least a century, if not more, into the future. And "chemical-based energy carrier" is a fancy word for fuel. If you look at the energy infrastructure that we have right now, over half of the energy we use is in chemical-based energy carriers. And we have both a physical infrastructure and kind of a lifestyle infrastructure, if you want to call it that, that requires transportable energy carriers. Electricity or batteries are really good for some applications. But they're not going… they're not going to solve all the problems. And so the question is really how do we make chemical-based energy carriers, or fuels, as efficiently as possible utilizing those waste streams? And then how do we use all the good work that people have done upstream and actually burn the fuels as efficiently as possible?
HOST: What are some of the results from the project so far?
JO: On the science side, we're very much midstream. We have been successfully burning hydrochars for a while now. My student has gotten in the lab and started to learn the microscopy. And there's been a lot of feedback on the combustion-to-synthesis side. In this next semester, we're really going to start focusing on the modeling side. Now that we have a baseline understanding of what's going on with the combustion so that we know what to target for, which chars we want to image, and then which ones we want to model and look deeper into.
The other exciting thing that's happening is I, with a couple of colleagues, have edited a book. It's called “Renewable Fuels: Sources, Conversion and Utilization.” It's published by Cambridge Press, and it's coming out in December 2022. I'm extremely excited about that book. Like I said, I think chemical energy carriers are going to be really important. And we want, we put together the book in such a way that people from all different parts of this problem could approach the books.
HOST: Thank you, Jacqueline, for spending time on Growing Impact and talking about your research.
JO: Thanks so much for having me. This was a really great opportunity. If folks want to learn more, they can visit my lab's website, which is https://sites.psu.edu/rfdl/, which stands for the Reacting Flow Dynamics Laboratory. Or shoot me an email (jxo22@psu.edu) I'd love to hear more.
HOST: This has been Season Three, Episode Six of Growing Impact. To learn more about IEE and to hear previous episodes, read podcast transcripts, and to see related graphics from Growing Impact episodes, please visit https://iee.psu.edu/news/podcast. Thank you for listening.
Jillian Goldfarb










