Have you ever wondered what happens to your body when you breathe in smoke? To understand the health effects of environmental exposure to inhaled toxic chemicals, we first need to be able to measure how much exposure a person has. This is important for both intentional exposures (such as smoking cigarettes or cannabis) and unintentional exposures (such as car exhaust or wildfire smoke).
Just like we need to know the correct dose of medicine to give a patient, we need to know the dose of an inhaled toxicant a person receives in order to make a definitive link between exposure and negative health outcomes.
Measuring exposure to inhaled chemicals
Measuring the dose of an inhaled chemical and connecting it to its source can be challenging. For example, there are over 7,000 chemicals in cigarette smoke. Many of those chemicals are found in other things like car exhaust, barbequed food, and cured meats, making it hard to separate cigarette exposure from other environmental exposures.
Nicotine might be an obvious choice for a marker of cigarette exposure, but if someone is using synthetic nicotine found in some e-cigarettes, it would be difficult to accurately tell their exposure to cigarette smoke specifically. In the case of tobacco smoke, tobacco-specific nitrosamines (TSNAs) were ultimately identified as a marker of exposure.
Identifying markers or chemicals specific to the source is the gold standard for understanding the negative health effects associated with inhalation from that source.

Free radicals as potential markers of exposure
While finding a single marker among a large group of chemicals can be difficult, understanding the chemistry behind how a source produces toxic chemicals can provide clues to potential markers. When items are burned (cigarettes, gasoline, forests, etc.), potentially hazardous compounds known as free radicals are produced as part of the combustion process.
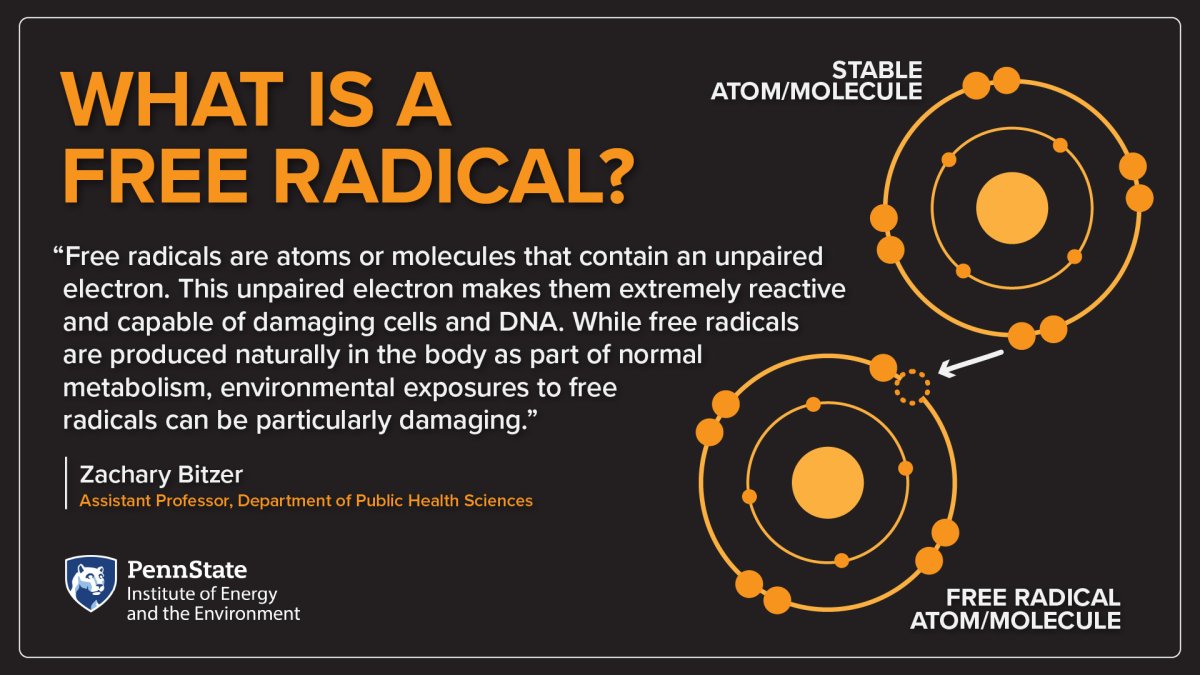
Free radicals are atoms or molecules that contain an unpaired electron. This unpaired electron makes them extremely reactive and capable of damaging cells and DNA. While free radicals are produced naturally in the body as part of normal metabolism, environmental exposures to free radicals can be particularly damaging.
By understanding the free radicals produced by a particular source, we can begin to identify unique radicals that may be produced.
Identifying free radicals
Free radicals are often understudied due to their transient nature and the challenges that come with detecting, measuring, and identifying them. They are often thought of as a single group of chemicals, but free radicals can be formed from many different molecules.
Identifying these free radicals can be done through a combination of electron paramagnetic resonance (EPR) and high-pressure liquid chromatography mass spectroscopy (HPLC-MS). Each radical structure produces a unique EPR trace or “fingerprint” that can be used to quickly identify the radical species. One advantage of this method is that while an air pollutant may have thousands of chemicals in it, the free radical population will be comparatively smaller.
Using free radicals to develop biomarkers of exposure
Our lab is currently using this approach to develop a biomarker specific to e-cigarettes. Many of the compounds found in an e-cigarette (propylene glycol, glycerin, flavorings) can be found in food products, beverages, and medications. Thus, using these compounds as a marker of exposure specific to e-cigarettes isn’t an option. E-cigarettes often contain nicotine, but many e-cigarette users also use cigarettes or other tobacco products, also making this marker not specific enough.
We have found that the free radicals produced by e-cigarettes are distinctly different chemically than those produced by conventional cigarettes. This relates back to the compounds found in the e-cigarette. While many people may consume food, drinks, or medication containing propylene glycol, glycerin, and flavorings, most people aren’t inhaling them after they’ve been heated. This makes them a particularly distinct marker for exposure. Our lab has shown that heating is a crucial component in the production of these free radicals. No heat, no free radicals. The number of free radicals produced by e-cigarettes also depends on the amount of propylene glycol and glycerin in the e-liquid. We’ve also shown that different flavor chemicals can increase and decrease the number of free radicals produced.
Since flavor chemicals differ from e-cigarette to e-cigarette, our research is focused on the two components found in every e-cigarette – propylene glycol and glycerin. With further research, we hope to identify the structures of these free radicals produced by these two chemicals and identify what molecules they interact with in the lung when inhaled, allowing us to develop a marker of exposure dose.
Benefits of using free radicals as markers of exposure
By using free radicals as a potential marker of exposure, we may be able to identify new markers of exposure more quickly and understand the health impacts of new and emerging environmental pollutants better. This can help us understand a given dose of that pollutant and better understand any negative health effects associated with that exposure.
Zachary Bitzer is a faculty member in the Institute of Energy and the Environment and an assistant professor in the Department of Public Health Sciences in Penn State's College of Medicine. His primary research interest is in environmental toxicology, including how toxins are produced, how they interact with human bodies, and novel biomarkers that could be developed to determine exposure levels.